You’re sitting on valuable silver trapped in lead-zinc ores. Traditional smelting loses up to 35% – heap leaching could recover more while cutting costs. Heap leaching extracts silver from lead-zinc ores by crushing to specific sizes (typically 6-25mm), stacking in permeable heaps, and applying cyanide solutions (NaCN 0.05-0.1%) at controlled pH (10-11) for 30-60 days. The method achieves 60-80% silver recovery at 50% lower costs than conventional processes.
What Should We Do When Extract Silver From Lead-Zinc Ore Using Heap Leaching?
How to extract silver from lead-zinc ore using heap leaching requires first determining whether the ore is sulfide or oxide. It is also crucial to identify whether the silver exists as easily leachable silver minerals—such as galena, native silver, and argentite—or as less soluble varieties like stibnite, sphalerite, selenite, or stibnite-copper-silver ore.
Ever dumped cyanide on worthless rock? Selection makes or breaks your operation. Heap leaching works best for oxidized lead-zinc ores with 100-500g/t silver, low sulfide content (<15%), and permeability >10cm/hr. Ideal ores show:
- Galena (PbS) grains fully oxidized to anglesite (PbSO₄)
- Iron oxide content <25% (avoids cyanide consumption)
- Clay content <10% (maintains heap permeability)
Ore Suitability Checklist:
| Parameter | Ideal Range | Rejection Criteria |
| Silver Grade | 80-800g/t | <40g/t unprofitable |
| Sulfide Content | <15% | >30% requires pretreatment |
| Clay Content | <10% | >20% causes ponding |
| Particle Size | 6-25mm | <5mm reduces percolation |
After determining the mineral phases, cyanide leaching conditions must be established. For oxidized zinc ores, zinc readily dissolves in cyanide solutions—particularly smithsonite (ZnCO₃), sphalerite (ZnO), and hydrated sphalerite (3ZnCO₃·2H₂O) dissolve exceptionally readily in cyanide solutions. This consumes large amounts of cyanide, and the resulting Zn(CN)₂ precipitates onto gold grain surfaces, hindering silver dissolution. When ore contains trace amounts of lead, it benefits the cyanidation of gold and silver, as lead neutralizes the detrimental effects of alkali metal sulfides in the cyanide solution. During the leaching of argentite (AgS), lead salts cause the S²⁻ ions in the generated Na₂S to precipitate as PbS, thereby enhancing silver dissolution in the cyanide solution. However, this effect does not apply to complex silver sulfide ores.
Excessive lead content adversely affects gold and silver leaching rates, which decrease as lime dosage increases.
Optimal heap leach conditions use:
- NaCN concentration: 0.05-0.1% (500-1000ppm)
- Lime-controlled pH: 10.2-10.8
- Application rate: 5-10L/hour/m²
- Leach cycle: 5 days wet/2 days dry

What is The Heap Leaching Procedure for Extracting Silver From Lead-zinc Ore?
1. Ore Preparation
The purpose of pretreatment is twofold: firstly, to achieve a certain particle size so that the cyanide leaching solution can fully contact the silver; and secondly, to improve the ore’s permeability so that the leaching solution can evenly pass through the ore heap.
Pretreatment methods include crushing to a suitable particle size for heap leaching and crushing followed by granulation for heap leaching. For ores generally suitable for heap leaching, crushing to a size between 10mm and 30mm is acceptable.
2. Heap Construction
(1) Construction of Heap Leaching Sites
Heap leaching sites are generally selected on gentle slopes near mining areas with convenient transportation. First, weeds and loose soil are removed, then the soil is compacted to form a foundation with a 3-5% slope, facilitating the gravity flow of leachate into the storage tank. Near the heap leaching site, lean solution ponds, precious solution ponds, and flood control facilities should be constructed.
(2) Heap Building
Heap building is a key step in heap leaching. The method of heap building affects the permeability of the ore pile and the uniform distribution of coarse and fine particles, directly influencing the silver leaching rate.
- Heap building can be done manually or mechanically.
- During heap building, it is important to avoid crushing the ore to prevent the formation of secondary slime, and to avoid localized compaction and particle segregation. This ensures a uniform particle size distribution, good water and air permeability of the heap, allowing cyanide solution and oxygen to flow throughout the heap and preventing channeling and blockage zones during leaching.
- Heap building methods include: partial heap building, layered heap building, and segmented layered heap building.
- The heap height for heap leaching depends on the scale of the operation, generally ranging from 2 to 5 meters.
(3) Spraying
Before spraying cyanide solution onto the ore heap, it should be washed with water and alkali. The cyanide solution should be sprayed only when the pH of the washing solution reaches 9.5–11.
Spraying Equipment: Uniform solution distribution is one of the important factors in improving the leaching rate. There are two methods of solution distribution: spraying and dripping. Regardless of the method, the leachate should cover the entire ore heap. The flow rate at all points on the surface should be uniform, minimizing evaporation and drift. The solution distribution system should be made of corrosion-resistant materials, such as polyvinyl chloride (PVC) plastic.
3. Leaching
The leaching including the following steps:
- Apply primary leach (0.1% NaCN) for 30 days
- Switch to secondary rinse (0.02% NaCN)
- Monitor pregnant solution (>10mg/L Ag)
(1) Primary Leaching
① Ore Washing
To reduce cyanide and alkali consumption, alkaline washing is performed before spraying cyanide solution onto the ore pile (if necessary, water washing precedes alkaline washing). Either a CaO aqueous solution or a NaOH aqueous solution can be used. Lime solution is inexpensive but requires extended leaching time and is prone to calcium scaling. Sodium hydroxide solution offers shorter leaching times but is costly. Once the pH of the leachate from the heap reaches 9.5–11 (typically after 3–5 days), spraying with a mixed solution of NaCN and alkali can commence.
② Spray Leaching
Throughout heap leaching, maintain optimal concentrations of cyanide, alkali, and oxygen.
Sodium cyanide concentration: Typically, the control leaching solution sodium cyanide concentration is between 0.02–0.1%.
NaCN concentration should be controlled in phases: typically starting at 0.08–0.1%, mid-stage at 0.05–0.06%, and late stage at 0.02–0.05%. During production, regularly measure cyanide concentration and leachate pH, adjusting promptly—typically 1–2 times daily.
③ Spray Intensity
Spray intensity is typically 10–20 liters/m².
Lower values apply to large, poorly permeable ore piles, while higher values suit smaller, more permeable piles. Continuous or intermittent cyanide solution spraying may be used; intermittent spraying facilitates oxygen replenishment and enhances gold/silver leaching. Spray for 2 hours, pause for 1 hour, then resume spraying.
When spraying alkaline cyanide solution, regularly inspect nozzles and pipelines for blockages. Address areas with insufficient coverage to ensure the solution reaches every location, guaranteeing silver leaching efficiency.
To prevent scaling in nozzles, pipelines, and ore piles, add appropriate scale inhibitors to the cyanide leaching solution.
Heap Leaching Duration
The required heap leaching time depends on factors such as ore properties and particle size, determined by testing. Typically, it ranges from 1 to 2 months, with longer durations possible up to 3 months. Spraying can be stopped when the gold grade in the leachate falls below 0.1 g/m³.
The leached gold and silver-bearing solution flows into the precious liquid pool. Gold and silver are recovered using methods such as carbon adsorption, zinc displacement, or direct electrowinning.
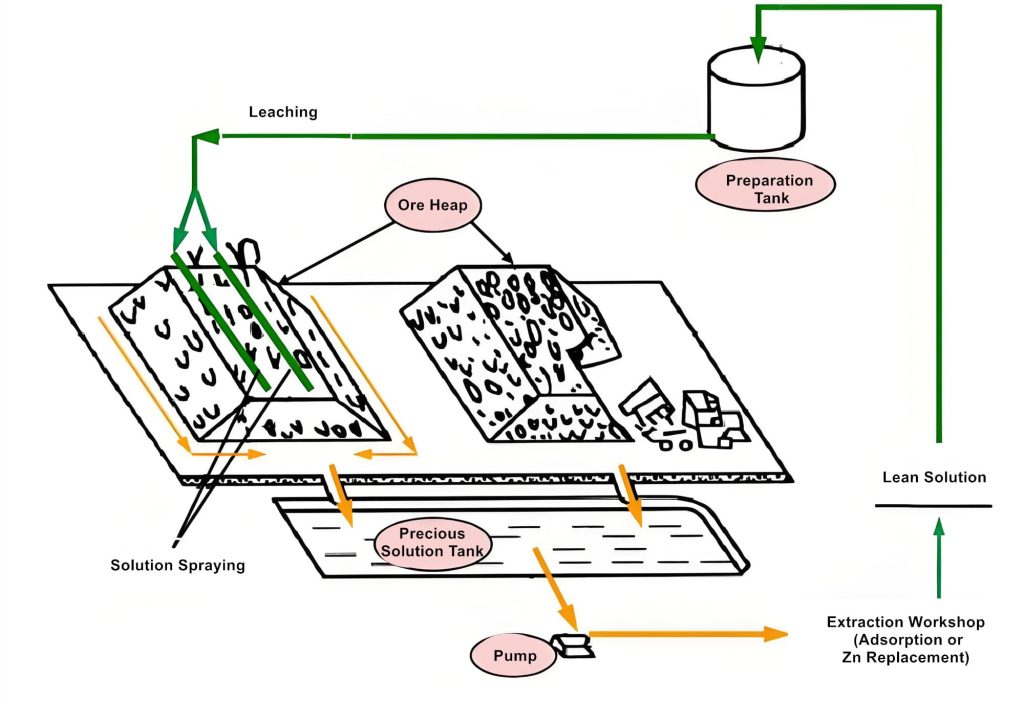
(2) Secondary Rinse
After heap leaching, the ore heap is washed with clean water, and the washing liquid is recovered and used to prepare a new leaching solution. The tailings are disinfected after washing, typically by spraying a bleaching powder solution. The washing water can flow into a flood control pond or lean solution pond, where it is treated to reduce the cyanide concentration to the national emission standard (<0.5 mg/L) before being discharged.
Factors affecting the heap leaching process include: cyanide concentration, oxygen content, pH value, spray intensity, ore properties, heap permeability, and leaching temperature.
To improve the leaching rate and extraction rate of gold and silver from the ore, research is currently underway in the following areas:
- Replacing air with O2. For example, introducing O2 for 36 days can increase the gold leaching rate from 82% with air to 91%.
- Using hydrogen peroxide or calcium peroxide to increase the oxygen content of the ore heap.
(3) Monitor Pregnant Solution
For precious metal solutions with high gold and silver content and large volumes, zinc displacement precipitation is generally used. Activated carbon adsorption is not recommended because silver competes with gold for adsorption, thus reducing gold adsorption capacity.
For mines with medium-scale heap leaching and moderate gold and silver content, activated carbon or resin adsorption-desorption-electrodeposition processes can be used to obtain precious metals.
For precious metal solutions with high gold and silver content (over 30-50 mg/L), gold and silver can be extracted directly by electrodeposition after clarification.
Activated carbon adsorption is simple to operate and has low investment costs, making it a widely used method in heap leaching.
When the silver content in the gold-bearing solution is high, sodium sulfide can be used to precipitate silver first, and the silver will precipitate as silver sulfide. Before the precious metal solution enters the adsorption process, the silver is removed from the solution by filtration (silver sulfide precipitation). This eliminates the possibility of high silver ion concentration affecting the adsorption of gold by activated carbon during carbon adsorption, increases the carbon’s gold adsorption capacity, and thus reduces the amount of desorption and electrodeposition required, lowering production costs.
Conclusion
Heap leaching offers a technically and economically viable method for recovering silver from lead-zinc ores. The key lies in precisely assessing ore characteristics upfront and optimizing process parameters. Every step—from selecting the right ore type (oxidized, low-sulfur, low-clay) to controlling leaching conditions (cyanide concentration, pH, irrigation rate), heap construction, and pregnant solution treatment—directly impacts silver recovery and operational costs.
Current technological advancements are moving toward greener and more efficient methods, such as oxygen-enriched leaching or peroxide-assisted oxidation. Future breakthroughs may also overcome pretreatment challenges associated with complex sulfide silver ores. For small- to medium-scale mines, activated carbon adsorption remains the most cost-effective option, while high-grade pregnant solutions allow direct electrowinning.
Remember: Heap leaching success = Ore suitability × Process precision × Real-time monitoring. With these three pillars in place, you can unlock hidden silver value from “waste rock” while minimizing costs and environmental impact.
