Manganese ore stands as a critical strategic resource in modern industries, playing an irreplaceable role in steelmaking, new energy batteries, chemical production, and other sectors. However, as high-grade manganese ore resources gradually deplete, the efficient processing of complex low-grade ores has become the industry’s core challenge. This article systematically analyzes manganese ore’s mineralogical classifications and industrial applications, delves into key technologies across the entire processing chain—including physical beneficiation, flotation concentration, and pyrometallurgical/hydrometallurgical pretreatment—and proposes innovative integrated mineral processing and metallurgical solutions tailored to China’s typical manganese ore characteristics of being “lean, fine-grained, and complex.” It provides technical pathway references for achieving efficient resource utilization and sustainable development in the industry.
Overview of Manganese Ore and Industrial Challenges
1. Mineralogical Classification and Industrial Applications
Manganese ore is a critical industrial metal resource widely used in metallurgy, chemicals, batteries, and other industries. Based on mineral composition, it can be categorized into:
- Oxide Manganese Ore(e.g., Pyrolusite MnO₂, Psilomelane (Ba,H₂O)₂Mn₅O₁₀) – Primarily used for electrolytic manganese and battery cathode materials (e.g., lithium-manganese oxides).
- Carbonate Manganese Ore(e.g., Rhodochrosite MnCO₃) – A key raw material in metallurgy for ferromanganese and silicomanganese alloys.
- Silicate Manganese Ore(e.g., Rhodonite MnSiO₃) – Requires specialized processing due to complex beneficiation.
- Sulfide Manganese Ore(e.g., Alabandite MnS) – Less common, mainly used in producing manganese sulfate for chemical applications.
Industrial Applications
- Steel Industry (70%+)– Manganese alloys enhance steel strength and wear resistance.
- Battery Industry– Lithium-ion batteries (e.g., NMC cathode materials), alkaline batteries (electrolytic manganese dioxide).
- Chemical Industry– Potassium permanganate, manganese sulfate for water treatment, fertilizers, and dyes.
- Other Uses– Aluminum alloy additives, magnetic materials, etc.
2. Industrial Challenges and Beneficiation Goals
Despite its wide usage, manganese ore extraction and processing face several challenges:
Industrial Challenges
- Low-Grade Ore Prevalence– High volumes of low-Mn (<15%) ore require efficient enrichment.
- Complex Mineralogy– Manganese often coexists with iron, silicon, and calcium, making separation difficult.
- Slime Interference– Fine-grained ores tend to slime, reducing recovery rates.
- Environmental Pressures– Hydrometallurgical methods (e.g., acid leaching) generate wastewater, demanding eco-friendly solutions.
Beneficiation Goals
- Maximize Recovery – Optimize gravity and magnetic combined processes.
- Reduce Impurities – Minimize Fe, P, and Si content to enhance metallurgical performance.
- Fine Particle Recovery– Advanced techniques like flotation and slime removal.
- Sustainable Processing– Promote low-pollution methods (e.g., bioleaching).

Chinese manganese ores are generally characterized by low grades, fine grain sizes, and complex compositions. The core objectives of mineral processing are:
- Grade Enhancement: To meet metallurgical (e.g., FeMn alloy) or chemical processing requirements.
- Impurity Removal: Strictly controlling the content of harmful impurities such as phosphorus (P) and iron (Fe).
- Slime Overcoming: Manganese oxide ores often contain significant amounts of ore slime, which severely disrupts mineral processing.
Fundamentals of Physical Beneficiation: Efficient Concentration and Pre-treatment
1. Washing & Screening: Key to Slime Removal
Oxidized manganese ores often contain substantial clay coatings due to weathering and leaching. Washing-screening is essential prior to beneficiation. Purpose: Utilize hydraulic scouring and mechanical scrubbing to remove slime, reducing clay content in feed materials to below 5%. Equipment: Trommel scrubbers (for moderately sticky slimes); Trough scrubbers (for highly viscous slimes). Core Technology- Hydraulic Scrubbing: High-pressure water jets (0.2–0.3 MPa) remove surface clay, achieving 50 t/h per unit capacity.
- Vibratory Screening: Dual-layer screens (upper 8–10 mm, lower 3–5 mm) enable three-stage separation.
- Slime Removal Efficiency: Eliminates >80% of -0.075 mm slime, increasing Mn grade by 3–5%.
| Ore Type | Recommended Equipment | Particle Size (mm) |
| Weathered Manganese Ore | Trough Washer + High-Frequency Screen | 30–0.15 |
| Sedimentary Manganese Ore | Spiral Washer + Trommel Screen | 50–0.074 |
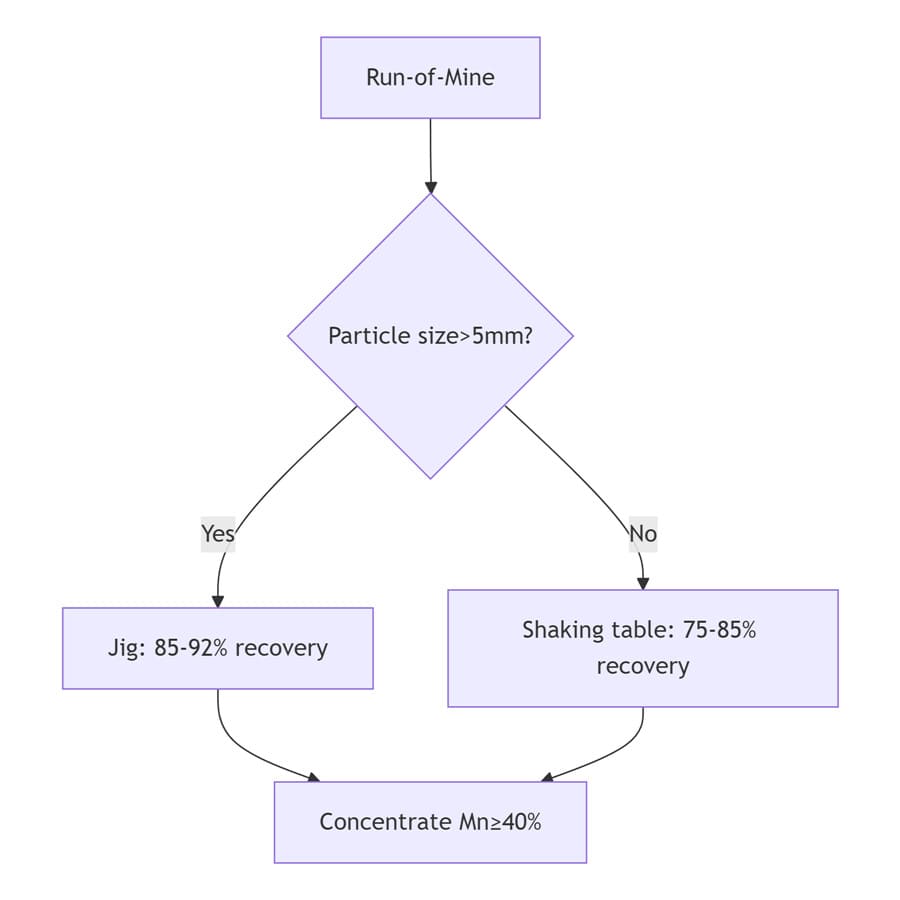
2. Gravity Separation: Cost-Effective Solution for Coarse Manganese
Gravity methods exploit density differences between pyrolusite and gangue minerals.
Applicability: Coarse-grained (0.5-10mm) oxidized manganese ores
Core Equipment: Jig concentrators (medium-coarse particles); Shaking tables (fine particles, as cleaning stage).
3. High-Intensity Magnetic Separation: Essential for Lean Oxidized Ores
Pyrolusite (MnO₂) exhibits weak magnetism. High-intensity magnetic separation (>1.2T) with strong field gradients effectively separates manganese from non-magnetic gangue (e.g., quartz, silicates).
- Wet HMS: High-gradient magnetic separators process fine slurries, critical for upgrading lean oxidized ores.
- Dry HMS: Serves as pre-concentration to reject low-moisture coarse waste.
Optimization Strategies
- Hybrid circuits: Washing-screening-HIMS combination achieves >90% recovery.
- Smart control: AI-based parameter adjustment maintains peak performance.
- Tailings utilization: >65% of magnetic tails convertible to construction aggregates.
Flotation Purification: Solving Carbonate and Slime Challenges
Flotation primarily treats finely-disseminated oxidized manganese and rhodochrosite (MnCO₃) ores, removing silicate and carbonate impurities.
- Rhodochrosite Flotation: Requires anion collectors (e.g., oleic acid, fatty acid soaps) under alkaline pH, with sodium silicate/hexametaphosphate as silicate depressants.
- Oxidized Ore Flotation: Complex reagent systems typically employ fatty acid collectors in weakly alkaline conditions to separate silicates/carbonates.
1. Core Difficulties in Mn Flotation
Carbonate Interference (MnCO₃ Dominant Ores)
Selectivity Issue: Similar surface properties between Mn-carbonates (rhodochrosite) and gangue carbonates (calcite/dolomite).
pH Sensitivity: Conventional collectors (fatty acids) non-selectively adsorb at pH 8–10.
Ultra-Fine Particle Problems (-20μm Slimes)
Mechanisms:
- Slime coating → Blocks collector adsorption
- High pulp viscosity → Impedes bubble-mineral attachment
- Excessive reagent consumption
2. Targeted Solutions & Process Innovations
Selective Depression for Carbonate Separation
| Strategy | Technical Approach | Expected Outcome |
| Staged pH Control | Acid pre-wash (pH 4–5) → Float Mn at pH 6.5–7.5 | Calcite depression ≥80% |
| Dual Depressant | Starch (200–500g/t) + Sodium silicate (300g/t) | Gangue carbonate recovery <15% |
Advanced Collectors for Fine Particles
Hydroxamate-based (e.g., Octyl hydroxamic acid):
- Chemisorption on Mn sites, reducing slime interference
- Dosage: 150–300g/t, 5–10°C lower than fatty acids
Nanobubble Flotation: 50–200nm bubbles enhance ultrafine Mn capture (↑Recovery 8–12%)
Pretreatment for Sliming Mitigation
Selective Agglomeration:
- Add hydrophobic binder (e.g., diesel 0.5kg/t) before grinding
- Forms 20–50μm Mn-bearing flocs, improving floatability
High-Gradient Magnetic Pre-concentration: Removes diamagnetic slimes (e.g., clays) prior to flotation.

Pyrometallurgical & Hydrometallurgical Pre-treatment: Tackling Refractory Ores
For ultra-fine, ultra-low-grade, or impurity-laden (especially iron) ores where physical methods fail, thermochemical approaches become necessary.
1. Reduction Roasting-Magnetic Separation
Targets: High-iron oxidized or low-grade refractory manganese ores.
Principle: Under reducing atmosphere, weakly magnetic hematite (Fe₂O₃) converts to strongly magnetic magnetite (Fe₃O₄), while manganese minerals remain weakly magnetic.
Separation: Low-intensity magnetic separation removes iron, boosting Mn grade.
Key Optimization Parameters
| Parameter | Optimal Range | Effect |
| Roasting Temperature | 750–900°C | >900°C → Sintering, ↓magnetism |
| Reductant Ratio | 8–15% (by ore weight) | Too low → incomplete reduction; too high → high energy use |
| Holding Time | 60–120 min | Too short → Fe incompletely reduced |
| Cooling Method | Quenching in inert gas (N₂) | Prevents Mn re-oxidation to MnO₂ |
2. Sulfuric Acid Leaching (Hydrometallurgy)
Suitable for: Ultra-low-grade carbonates or high-purity manganese salt production from oxidized ores.
Principle: Selective manganese dissolution via dilute acid (e.g., H₂SO₄), followed by solvent extraction/precipitation for recovery.
Advantage: Eliminates phosphorus/silicon impurities, yielding high-value chemical products.
Key Process Optimizations
| Challenge | Solution |
| High Acid Consumption (Ca/Mg interference) | Calcination Pretreatment (500°C) → Converts CaCO₃ to CaO |
| Slow MnO₂ Leaching Kinetics | Microwave Activation (enhances penetration) or SO₂ Reduction |
| Difficult Fe/Mn Separation | Solvent Extraction (D2EHPA) selectively extracts Mn |
Integrated Flowsheets & Development Trends
Industrial applications rarely employ single methods, instead customizing multi-stage circuits based on ore complexity and economics.
Oxidized Ore Flow: Washing → Jigging → HMS Cleaning → Flotation
Carbonate Ore Flow: Crushing → Grinding → Roughing/Cleaning Flotation
Refractory Ore Flow: Washing → Roasting-Magnetic Separation → Leaching
Manganese beneficiation constitutes a sophisticated system demanding deep ore characterization expertise and flexible integration of physical, magnetic, and chemical techniques for efficient, value-added recovery.
Conclusion
Today, the global manganese industry faces dual pressures of declining resource grades and tightening environmental constraints, driving technological innovation toward smarter, finer, and greener beneficiation solutions. From advancements in high-pressure water jet washing equipment to industrial applications of cutting-edge technologies like nanobubble flotation, and AI-controlled magnetic separation-roasting hybrid systems, manganese ore processing has evolved into a multidisciplinary field marked by integration. Looking ahead, breakthroughs in highly selective reagent systems, optimized short-flow process designs incorporating multiple techniques, and stepwise recovery of valuable components from tailings will open new avenues for the ultimate goal of “zero waste” manganese resource utilization—bolstering raw material security for the new energy and advanced manufacturing industries.
